clonoSEQ is now covered by Medicare for MCL patients.
It is the first Medicare-reimbursed MRD test for patients with MCL and is clinically available as a CLIA-validated LDT.
For more information about incorporating clonoSEQ into your clinical practice for MCL patients, click here.
Mantle Cell Lymphoma
clonoSEQ allows for precise, less toxic assessment of disease burden in MCL, so you can manage patients with confidence
CLIA-validated in peripheral blood and bone marrow
In mantle cell lymphoma (MCL), relapse is common due to the aggressiveness of the disease and the limitations of treatment. Additionally, imaging exposes patients to radiation and may increase anxiety in cases of false positivity. clonoSEQ addresses these concerns by complementing imaging with a minimally invasive, blood-based test that detects potential relapse early—helping you make more informed treatment decisions.
clonoSEQ can help you detect potential relapse early so you can start planning next steps
Predict long-term outcomes1,2
Understand your patients’ chances of progression-free survival (PFS).
clonoSEQ MRD negativity at 10-6 was a better predictor of favorable patient outcomes compared with clonoSEQ MRD negativity at 10-5. The deep sensitivity of MRD detection by clonoSEQ predicts long-term outcomes for your patients.1


All patients who were MRD positive 6 months post-end of therapy (EoT) eventually experienced relapse.
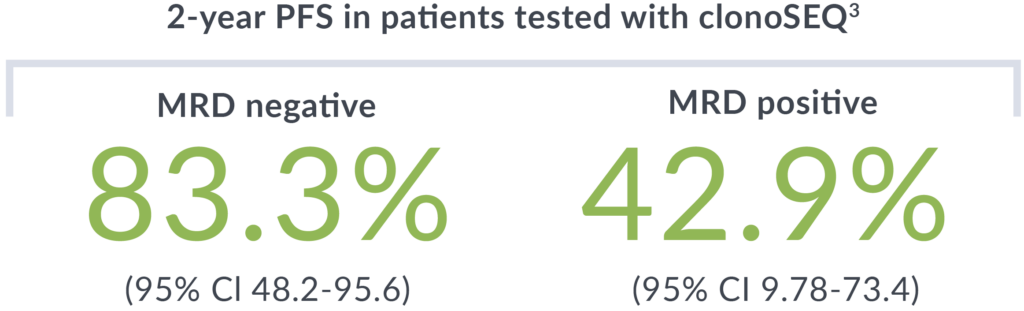
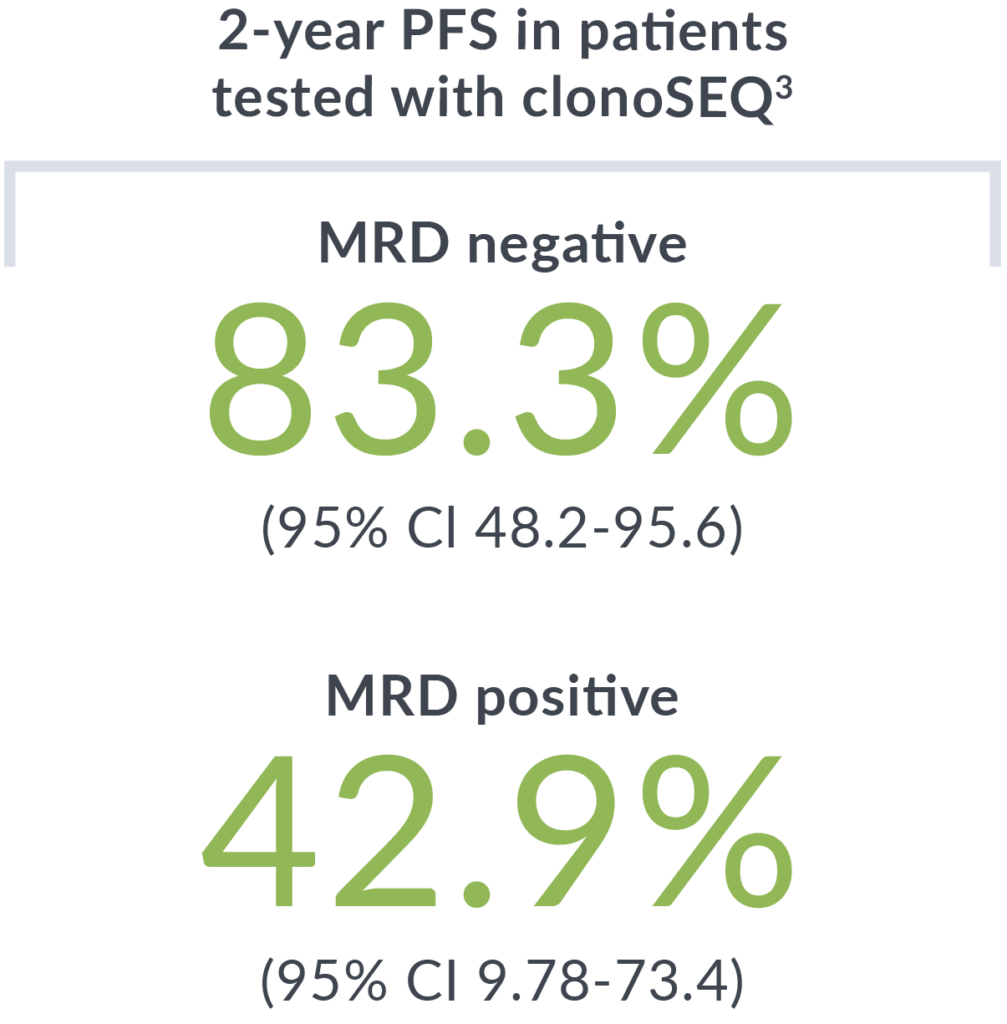
Patients were tested with clonoSEQ after their second cycle of bendamustine/obinutuzumab (BO). Evaluation of PFS post-chemotherapy showed similar outcomes regardless of receiving maintenance therapy.
Detect relapse early
Multiple studies show that clonoSEQ can help detect potential relapse weeks to months ahead of imaging.
92%
of post-frontline patients had relapse detected earlier than imaging by clonoSEQ. Relapse was detected a median of 34 days (range of 0-24 months) prior to a positive PET/CT result.2
of post-frontline patients had relapse detected earlier than imaging by clonoSEQ. Relapse was detected a median of 34 days (range of 0-24 months) prior to a positive PET/CT result.2

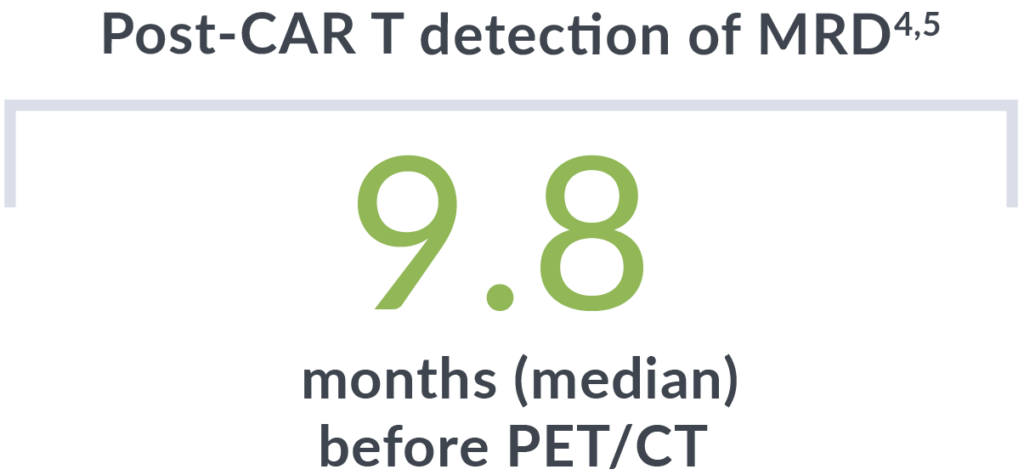
In addition to detecting relapse early, all patients who were MRD negative 3 months post-CAR T-cell therapy did not relapse (median follow-up of 14 months).4
clonoSEQ gives you clarity and confidence to help you optimize patient care
Inform changes to therapy
In recent studies, newly diagnosed MCL patients could transition from frontline therapy to surveillance if they had a complete response and were MRD negative by clonoSEQ.3,6,7
With subsequent blood-based MRD monitoring, patients were re-treated in the event of clinical progression, or if MRD was detectable at >10-6.2,7
“Our frequent testing for MRD [with clonoSEQ] during induction allowed for the modification of the protocol to allow patients to transition to maintenance sooner than originally planned.”
— Phillips et al
clonoSEQ can help you decide when to refer patients to an academic center for additional lines of treatment
Gain insight for post-CAR T-cell therapy treatment decisions.
In recent studies of CAR T-cell therapy for relapsed/refractory MCL patients, MRD negativity with clonoSEQ was associated with durable response, better PFS and overall survival outcomes, and higher levels of CAR T-cell expansion.4,5,8,9
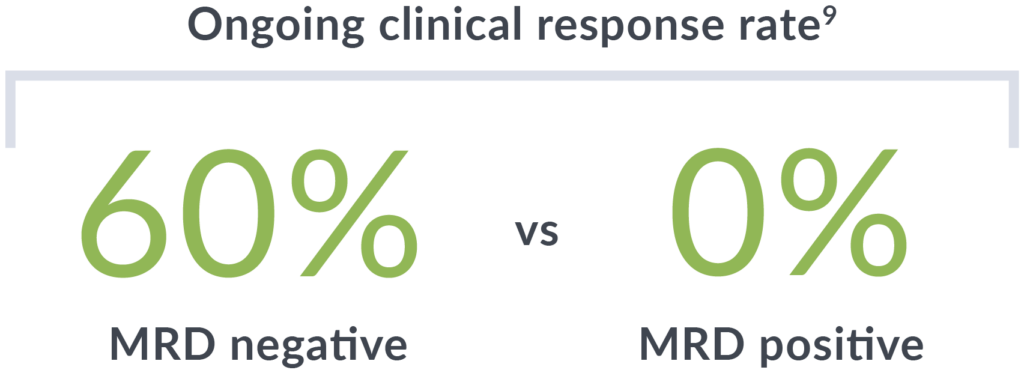
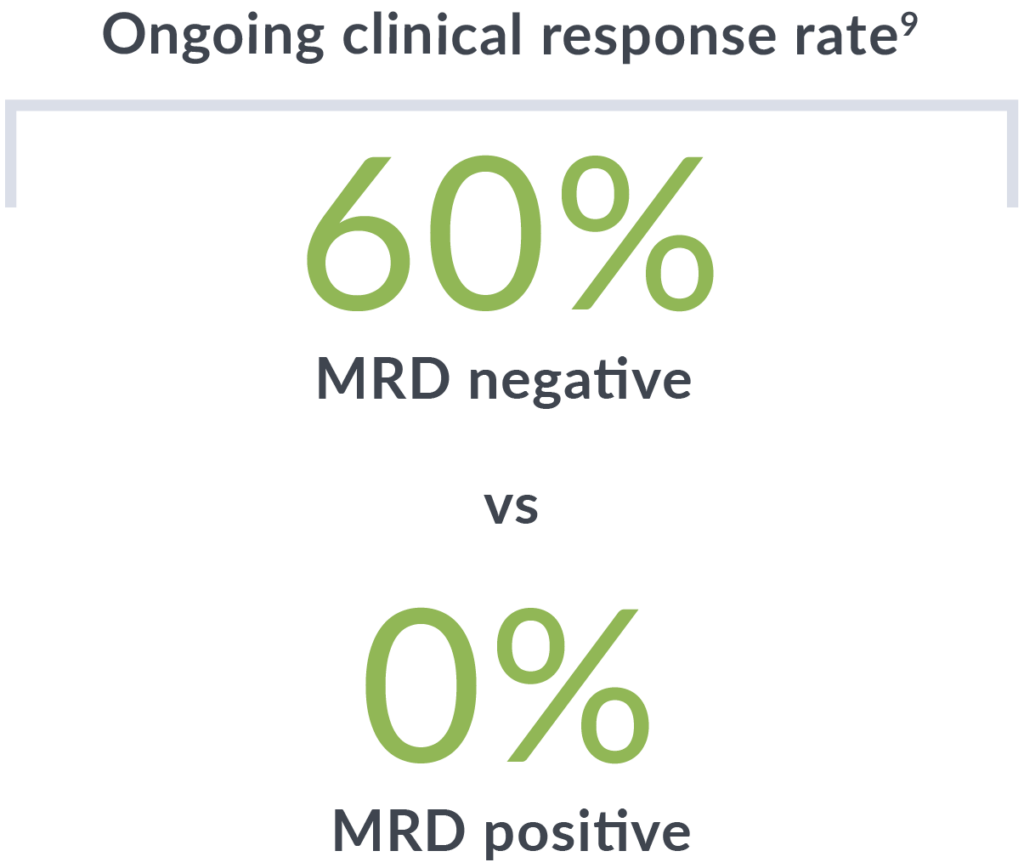
clonoSEQ MRD status at 6 months after treatment was correlated to ongoing clinical response (median follow-up of 3 years).
clonoSEQ can help you monitor your patients’ progress, so you can make the best decisions for their lives and health
Complement or reduce imaging
clonoSEQ can spare your patients from frequent PET/CT scans.
Repeated imaging may lead to overexposure to radiation10; clonoSEQ eases that concern by helping you assess disease burden throughout treatment with a blood-based test.
clonoSEQ MRD results are strongly concordant with PET/CT, without the related toxicities. 86% (95/111) of the time results matched between clonoSEQ MRD testing at 10-5 and PET/CT.1 Some patients who were MRD positive by clonoSEQ but PET/CT negative eventually relapsed.
“Several patients were able to enjoy 2- to 4-year intervals without any surveillance imaging, while still getting reassurance every few months from the negative clonoSEQ test.”
— Rezazadeh et al
clonoSEQ can complement imaging with a simple and accurate blood-based test
Leverage the clinical insights of clonoSEQ MRD testing
across treatment phases in MCL
Clinical practice guidelines and clinical trial data support regular MRD testing at multiple timepoints during and after treatment.
Induction
Predict long-term outcomes and assess the need for additional frontline therapy3,6
Maintenance
Monitor subtle changes in disease burden over time and detect potential relapse early5
Surveillance
Detect potential recurrence early with a simple, blood-based test5
Second-Line and Beyond
Assess response to therapy to inform subsequent treatment decisions and timing4
Coverage throughout a patient’s treatment journey
clonoSEQ is well covered, regardless of a patient’s insurance.
- National Medicare coverage for MCL patients at multiple testing timepoints with most patients paying $0 out-of-pocket
Ready to get started with clonoSEQ?
ctDNA MRD Testing Now Included in DLBCL Clinical Practice Guidelines
Clinical practice guidelines now include ctDNA MRD testing after frontline therapy for PET-positive DLBCL patients.
clonoSEQ is the only commercially available, Medicare-covered, ctDNA MRD test for DLBCL patients.
About the studies
Frontline Therapy
A phase 2 study of 47 previously untreated, high-risk MCL patients investigated use of sequential immunochemotherapy incorporating lenalidomide. Serial MRD monitoring with clonoSEQ was done at multiple timepoints, including 6 months post-EoT.
A phase 2 study of 21 previously untreated MCL patients who received 2 cycles of bendamustine and obinutuzumab with median follow-up of 43.9 months. MRD testing with clonoSEQ was performed after cycle 2 induction and post-consolidation to evaluate PFS and response rates at each timepoint.
Surveillance
A multicenter, open-label, nonrandomized, single-arm study evaluated lenalidomide, rituximab, and venetoclax triplet therapy in 28 patients with untreated MCL. MRD testing with clonoSEQ was performed at baseline and after cycles 3, 6, 9, and 12 during induction, and thereafter yearly during maintenance. Patients could transition to maintenance after cycle 6 of induction therapy if they were found to be in CR and were MRD negative by clonoSEQ.
A phase 2 trial of zanubrutinib, obinutuzumab, and venetoclax (BOVen) in patients with treatment-naïve, TP53-mutant MCL. After 24 cycles, Zanu and Ven could be discontinued if MRD negativity (<10-6) and CR was achieved. Patients could be retreated if clinical progression or MRD positivity (>10-6) with clonoSEQ were detected.
A study evaluating 21 adult patients with previously untreated transplant ineligible MCL reported correlation between PFS and PB MRD testing after C2 induction therapy represented a potential new prognostic marker of MCL behavior, and may be an important early disease indicator in development of risk-adapted therapies.
A retrospective analysis of 31 low-to-intermediate risk patients who underwent first-line treatment for MCL and were then monitored post-treatment using clonoSEQ. Median follow-up at 5 and 10 years evaluated progression-free survival.
Second-Line and Beyond
In the ZUMA-2 study of R/R MCL patients who received CAR T-cell therapy, 29 of 74 participants (39%) were serially monitored with MRD testing with clonoSEQ (10-5) as an exploratory analysis at baseline and months 1, 3, and 6 post-treatment.
A sub-analysis in ZUMA-2 of 27 patients post-treatment showed predictive performance of MRD measured at months 1, 2, and 6 in estimating relapse potential.
A phase I/2 single center, prospective trial evaluating dual targeted lentiviral anti-CD20/anti-CD19 for patients with R/R B-cell NHL, including a cohort of MCL patients. MRD was measured with clonoSEQ at a median of 28 and 90 days post-treatment.
A phase 1/2 single center, prospective trial evaluating LV20.19 CAR-T cells in 17 adult patients with R/R MCL reports and concludes that targeting CD20 and CD19 with CAR-T cells may improve outcomes in patients with relapsed, refractory MCL. Initial clonoSEQ MRD assay performed within the first 90 days of LV20.19 CAR T (n=13) was negative in 10 pts (77%).
Complement to Imaging
There were 111 coincident testing instances of PET/CT results and MRD status at 10-5 in the study from Epstein-Peterson et al, of which most (86%) were concordant: 6 as PET positive/MRD positive and 89 as PET negative/MRD negative. 16 coincident tests showed discordant results: 5 PET positive/MRD negative and 11 PET negative/MRD positive.
This page is intended for a US-based audience.
clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect measurable residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). Additionally, clonoSEQ is available for use in other lymphoid cancers and specimen types as a CLIA-validated laboratory developed test (LDT). To review the FDA-cleared uses of clonoSEQ, visit clonoSEQ.com/technical-summary.
References:
- Epstein-Peterson Z, et al. Haematologica. 2024;109(4):1149-1162.
- Rezazadeh A, et al. Clin Lymphoma Myeloma Leuk. 2023;24(4):254-259.
- McQuinn D, et al. Abstract 4407. Presented at: ASH Annual Meeting; December 2023; San Diego, CA.
- Shah N, et al. Abstract 1024. Presented at: ASH Annual Meeting; December 2023; San Diego, CA.
- Ananth S, et al. Abstract 1673. Presented at: ASH Annual Meeting; December 2023; San Diego, CA.
- Phillips T, et al. Blood Adv. 2023;7(16):4518-4527.
- Kumar A, et al. Abstract 738. Presented at: ASH Annual Meeting; December 2023; San Diego, CA.
- Wang M, et al. N Engl J Med. 2020;382(14):1331-1342.
- Wang M, et al. J Clin Oncol. 2023;41(3):555-567.
- Al Tabaa Y, et al. Cancers (Basel). 2021;13(20):5222