The clonoSEQ ctDNA MRD test for DLBCL now provides 7- fold greater sensitivity, with no loss of specificity or accuracy.
clonoSEQ is the only commercially available, Medicare-covered, ctDNA MRD test for DLBCL patients. Adaptive is committed to continually advancing MRD testing, providing the gold standard to inform patient care. To learn more about this development, please reach out to your local Adaptive representative or email us at Med Info.
Diffuse Large B-cell Lymphoma
clonoSEQ allows you to keep a close watch on disease progression in DLBCL
CLIA-validated in peripheral blood and bone marrow
Most DLBCL patients achieve remission after first-line therapy. But disease recurs in up to 40% of those patients, partly due to the persistence of low levels of measurable residual disease (MRD) which aren’t readily detected by standard imaging.1,2 For those who require second-line therapy, there are advanced treatments available which may still provide deep responses.
Regular clonoSEQ MRD testing during and after treatment can help maximize insights into long-term patient outcomes
Illuminate patient prognosis
clonoSEQ does more to predict long-term outcomes.1,3
In a retrospective study, circulating tumor DNA (ctDNA) was measured via clonoSEQ to assess response in previously-untreated DLBCL patients who were treated with etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) with or without rituximab.
5-year time to progression1
80.2%
of patients MRD negative by ctDNA did not progress
(95% CI 69.6-87.3)1
41.7%
of patients MRD positive by ctDNA did not progress
(95% CI 22.2-60.1)1
Patients who were MRD negative during treatment had a significantly improved progression-free survival (PFS) for up to 20 years1
Clarity beyond imaging
Surveillance monitoring with clonoSEQ can detect relapse earlier.
An inherent challenge with PET and/or CT scans in surveillance is their qualitative nature, which can lead to difficulty understanding the true magnitude of changes in disease burden, as well as radiation exposure1 and false positives due to inferior specificity.4,5
Earlier detection of disease:
In a study, clonoSEQ detected returning disease a median of 3.5 months (range 0-200 months) before imaging.1
Fewer false positives:
In separate studies, clonoSEQ had a positive-predictive value (PPV) of 88.2%; imaging had a PPV of <40%, meaning most positive PET/CT scans were not indicative of relapse.1,6,7
clonoSEQ detects disease at levels imaging cannot1,4,5
Future focused
clonoSEQ helps determine the likelihood of recurrence after chimeric antigen receptor (CAR) T-cell therapy.8
In a prospective multicenter study, the prognostic value of clonoSEQ was seen after CAR T-cell therapy (axicabtagene ciloleucel) in relapsed/refractory (R/R) DLBCL patients. MRD was assessed at week 1, day 28, and at/before month 3.
clonoSEQ predicted response as soon as 1 week after CAR-T infusion and found8:
70%
of durably responding patients were MRD negative
94%
of patients had detectable MRD at or before radiographic relapse
Only 13% of patients with progressive disease were MRD negative.
MRD Status (ctDNA) by clonoSEQ at day 288

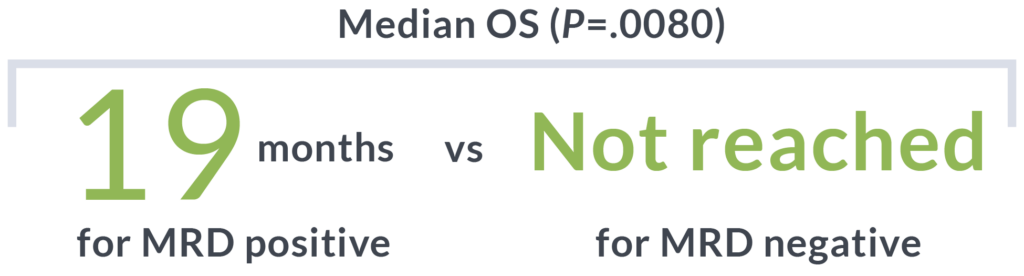

100%
of durably responding patients had MRD-negative ctDNA and did not relapse.8
clonoSEQ is predictive of sustained PFS with bispecific antibody therapy.
In a study on the efficacy of bispecific antibodies, clonoSEQ revealed9:
- Of the patients who were MRD negative via ctDNA during treatment, an estimated 78.7% of these patients remained MRD negative at 6 months
- Patients who achieved MRD negativity during treatment experienced longer PFS than those who were MRD positive
clonoSEQ can help predict long-term outcomes of the latest therapeutic advances in DLBCL9
Supporting decisions
clonoSEQ has utility before and after transplant.10,11
In a study of relapsed/refractory (R/R) DLBCL patients who underwent autologous stem cell transplant (ASCT), clonoSEQ was used to measure MRD in patient apheresis stem cell (ASC) samples pre-transplant, and from blood samples post-transplant.10,11
Prognostic value:
MRD was detected in 23% of the ASC samples pre-transplant, and was associated with shorter PFS and overall survival (OS) compared with those ASC samples that were MRD negative by clonoSEQ.11
Molecular relapse:
Median lead time from MRD detection post-transplant to clinical relapse was 62 days (range: 0-518 days).11
5-year outcomes by MRD status based on pre-transplant ASC samples
PFS at 5 years (P<.0001)11
53%
MRD-negative patients
13%
MRD-positive patients
(HR, 2.9; 95% CI: 1.7-5.0)
OS at 5 years (P=.05)11
68%
MRD-negative patients
52%
MRD-positive patients
(HR, 2.0; 95% CI: 1.01-4.0)
Detection of MRD by clonoSEQ before transplant is associated with poorer outcomes11
Convenience without compromise
clonoSEQ ctDNA assessment for DLBCL is available as a simple, minimally-invasive blood-based test that allows for more frequent testing and fewer bone marrow (BM) draws.
Leverage the clinical insights of clonoSEQ MRD testing across treatment phases in DLBCL
Clinical trial data support regular MRD testing at multiple timepoints during and after treatment.
Frontline
Evaluate response to therapy and plan next steps1,7
Surveillance
Detect low levels of disease resurgence sooner than imaging so you can discuss the next course of action1
Second-Line and Beyond
Gain potential lead time for planning next steps, whether it be CAR T-cell therapy, bispecifics, or ASCT8,10,11
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Guidelines include ctDNA MRD testing post-frontline therapy for PET-positive DLBCL patients.12
Coverage throughout a patient’s treatment journey
clonoSEQ is well covered, regardless of a patient’s insurance.
- National Medicare coverage for DLBCL patients at multiple testing timepoints with most patients paying $0 out-of-pocket
- Positive coverage policies from the largest national private insurers*
Ready to get started with clonoSEQ?
ctDNA MRD Testing Now Included in DLBCL Clinical Practice Guidelines
Clinical practice guidelines now include ctDNA MRD testing after frontline therapy for PET-positive DLBCL patients.
clonoSEQ is the only commercially available, Medicare-covered, ctDNA MRD test for DLBCL patients.
CT, computerized tomography; PET, positive emission tomography.
*Medicare Advantage patients may have out-of-pocket costs.
About the studies
Frontline
A retrospective study measured cell-free ctDNA (serum) in DLBCL patients who received EPOCH (4-component chemotherapy regimen), with or without rituximab. Monitoring of ctDNA by clonoSEQ was done on serum samples taken during treatment (day 1 of cycle 3) and with serial monitoring starting from end of treatment (EoT) until disease progression and only included patients in complete remission at the EoT. 1,3
In this prospective study of 2 disparate cohorts of DLBCL patients, treatment was at the discretion of the investigators and therefore varied among patients. 311 blood samples across 75 DLBCL patients were used to explore the clinical utility of clonoSEQ (previous iteration named Lymphosight) for detection of molecular disease from plasma ctDNA and peripheral blood (PB) circulating tumor cells for disease monitoring, compared with PET/CT and ultimate clinical outcomes. PB samples were defined as occurring in any of 4 time points: pre-treatment, during treatment, surveillance, and progressive disease.1,3
Surveillance
A retrospective study of 118 patients diagnosed with DLBCL to establish the cost and clinical outcomes. Costs incurred specifically for DLBCL from the date of complete response (CR) after completion of standard chemotherapy through the subsequent 3 years were calculated.4
Post-CAR T-cell therapy
This study assessed the prognostic value of ctDNA before and after axicabtagene ciloleucel infusion. ctDNA was available for analysis in 60 patients following lymphodepleting chemotherapy either until progression or 1-year following infusion. MRD levels were determined in the 30 patients who progressed and 30 who responded.7
This study was comprised of 116 patients (75 DLBCL, 34 transformed indolent lymphoma, 7 primary mediastinal large B-cell lymphoma) who underwent ASCT. Pre-transplant MRD was assessed in ASC samples (n = 98 patients) and plasma/peripheral blood mononuclear cell samples (n = 13 patients). Post-transplant MRD was assessed in plasma/peripheral blood mononuclear cell samples (n = 60 patients).10
Bispecific antibody treatment
In the dose-expansion cohort of a phase I/II study, adults with R/R DLBCL and at least 2 prior therapy lines received subcutaneous epcoritamab in 28-day cycles until disease progression or unacceptable toxicity. MRD was an exploratory analysis and samples were collected at protocol-specified time points on day 1 of cycles 1, 3, 5, 7, 10, and 13 (during treatment), and then every 6 (±1) months after 1 year, for up to 3 years.8
This page is intended for a US-based audience.
clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect measurable residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). Additionally, clonoSEQ is available for use in other lymphoid cancers and specimen types as a CLIA-validated laboratory developed test (LDT). To review the FDA-cleared uses of clonoSEQ, visit clonoSEQ.com/technical-summary.
References:
- Roschewski M, et al. Lancet Oncol. 2015;16(5):541-549.
- Hu R, et al. Curr Oncol Rep. 2019;21(5):44.
- Kurtz D, et al. Blood. 2015;125(24):3679-3687.
- Suh K, et al. Korean J Intern Med. 2019;34(4):894-901.
- Carr R, et al. J Nucl Med. 2014;55(12):1936-1944.
- Avivi I, et al. Am J Hematol. 2013;88(5):400-405.
- El-Galaly T, et al. Leuk Lymphoma. 2011;52(4):597-603.
- Frank M, et al. J Clin Oncol. 2021;39(27):3034-3043.
- Thieblemont C, et al. J Clin Oncol. 2023;41(12):2238-2247.
- Merryman R, et al. Blood. 2020;136:22.
- Merryman R, et al. Blood Adv. 2023;7(17):4748-4759.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-cell Lymphomas (version 1.2025). © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed January 16, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.