Understanding Your Report
Let’s take a closer look at the clonoSEQ reports
Your doctor will likely order clonoSEQ MRD testing at several points throughout your treatment journey to assess your response to therapy and inform the clinical decisions you make together. Your MRD test results will be delivered to your doctor in the form of a clonoSEQ report. There are two types of clonoSEQ reports you may review with your doctor.
Be sure to talk with your doctor about the optimal timing for MRD testing with clonoSEQ
Sample Clonality (ID) Report
Tap numbers on the report for more information.
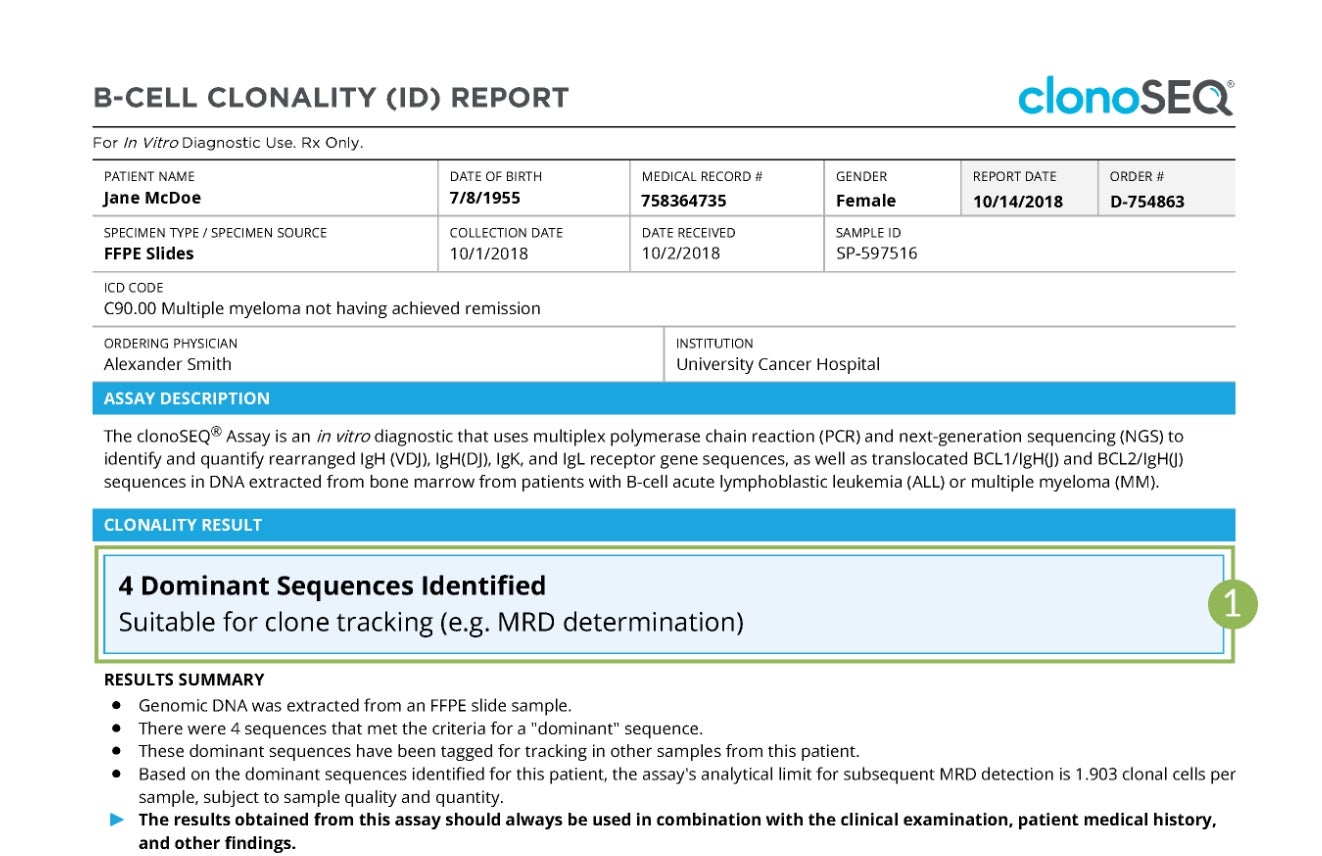
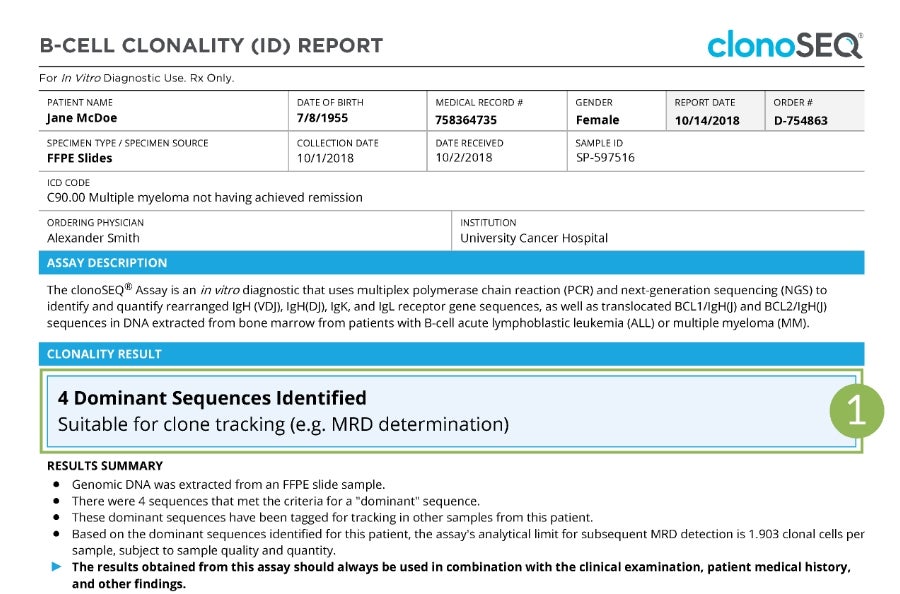
Clonality (ID) Status
The number of trackable DNA sequences that were identified. You may have one DNA sequence or multiple DNA sequences.
In rare cases, a dominant sequence may not be identified. If this occurs, we will work with your doctor to identify other specimen options.
Talk to your doctor about ordering your Clonality (ID) Test upfront, so you can have a baseline for your future MRD tests. Ask your doctor how often you will be testing with clonoSEQ and receiving your reports.
Sample Tracking (MRD) Report
Tap numbers on the report for more information.
Your MRD Status
A positive (+) result means residual disease was detected. A result of zero means residual disease was not detected. Each report will provide your updated MRD status.* You can gain valuable insights about your cancer regardless of your result.
Talk with your doctor about your MRD status to better understand what your result means for you and your treatment plan.
*False-positive or false-negative results may occur for reasons including, but not limited to: contamination, technical, and/or biological factors.
MRD Level
This number shows the amount of cancer present in your sample when it is taken. Your doctor can help put this number into context based on your current phase of care and treatment goals.
MRD Trends
A graph will show any changes detected in your MRD level over time. Watching these changes may help you and your doctor better understand your response to treatment and track any changes in your cancer levels.
This page is intended for a US-based audience.
clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect measurable residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). Additionally, clonoSEQ is available for use in other lymphoid cancers and specimen types as a CLIA-validated laboratory-developed test (LDT). To review the FDA-cleared uses of clonoSEQ, visit clonoSEQ.com/technical-summary.